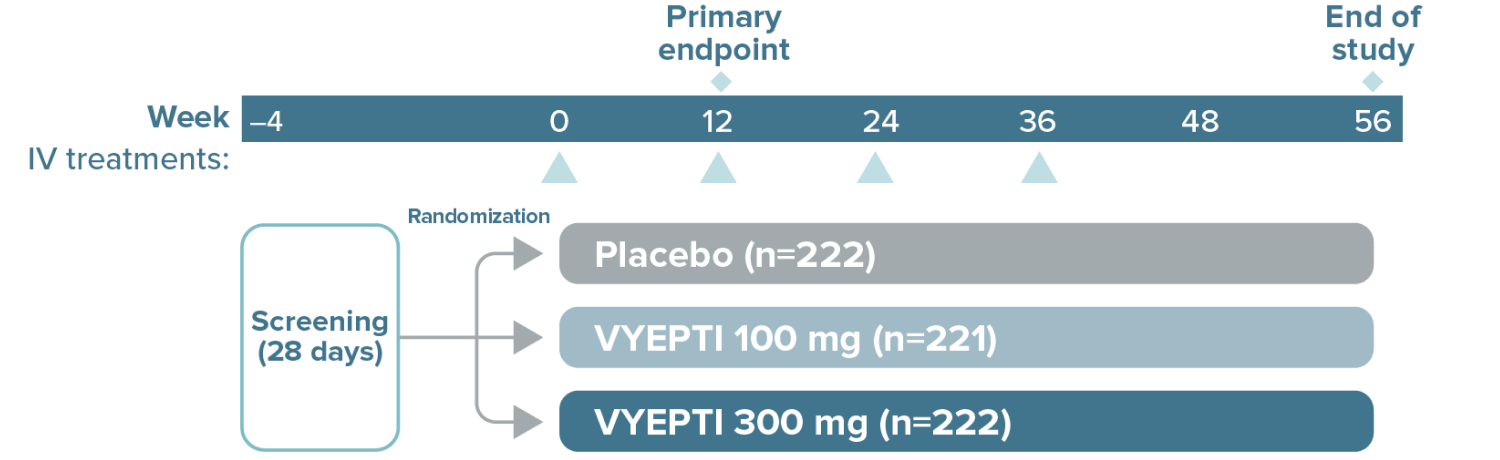
Review efficacy data from the VYEPTI pivotal trials
- PRIMARY ENDPOINT
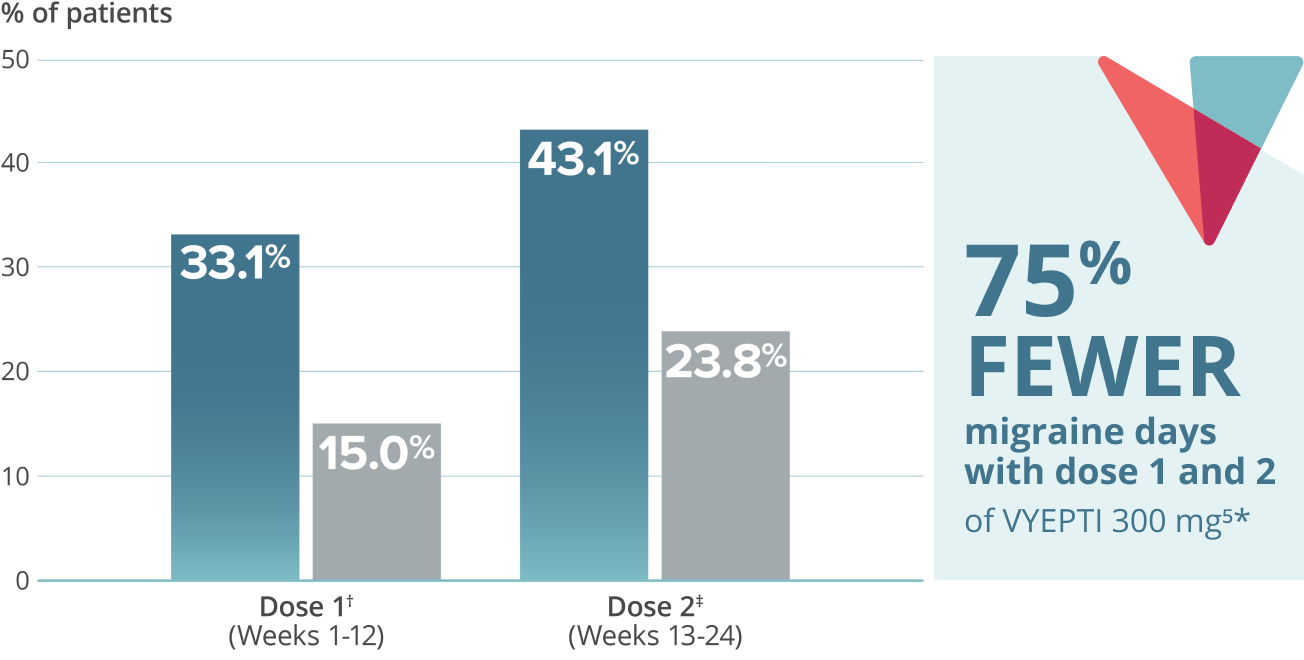
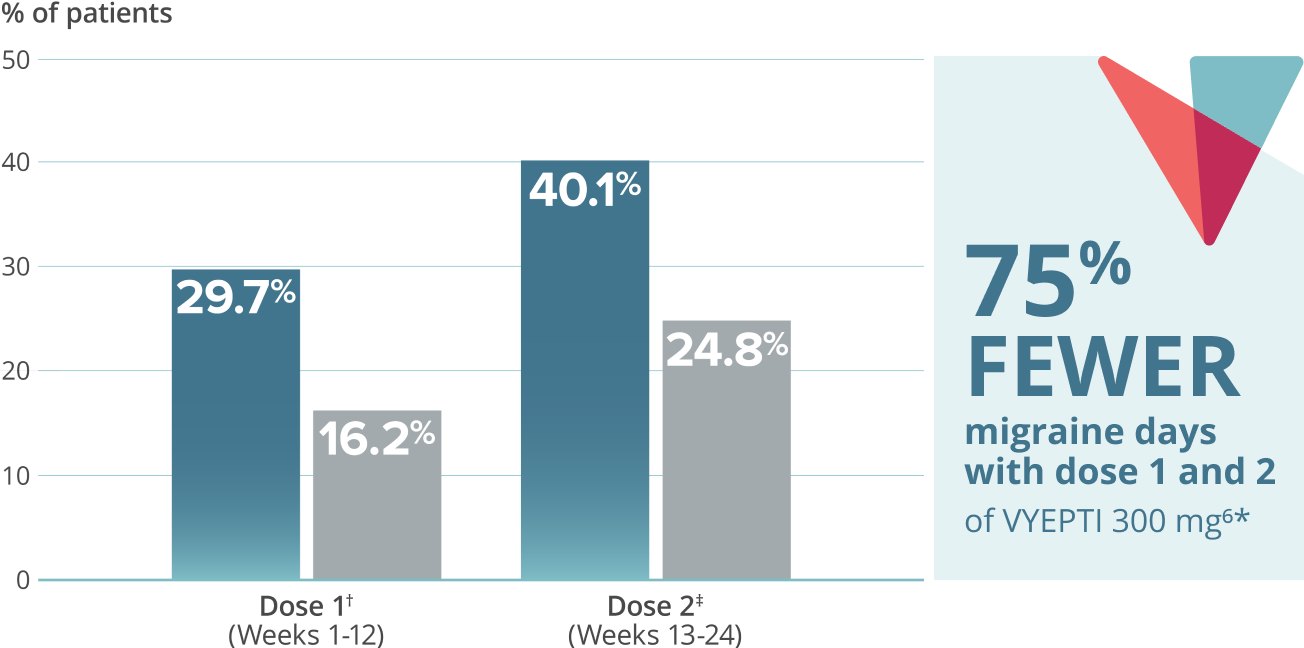
- 75% FEWER MIGRAINE DAYS
- FAST ONSET, SUSTAINED PREVENTION
- REDUCTION IN ACUTE MED USE
- HEADACHE IMPACT TEST (HIT-6)
The study included:
- Patients using an established stable regimen of acute migraine or headache prevention medication (except onabotulinumtoxinA)
- Patients with a dual diagnosis of chronic migraine and medication overuse headache attributable to acute medication overuse (triptans, ergotamine, or combination analgesics >10 days per month)
The study excluded:
- Patients using opioids or butalbital-containing products >4 days per month
- Patients with a history of:
- Cardiovascular disease (hypertension, ischemic heart disease)
- Neurological disease
- Cerebrovascular disease
The study included:
- Patients using concurrent acute migraine or headache medications, including migraine-specific medications (eg, triptans, ergotamine derivatives)
The study excluded patients with a history of:
- Cardiovascular disease (hypertension, ischemic heart disease)
- Neurological disease
- Cerebrovascular disease
≥75% migraine responder rate5*
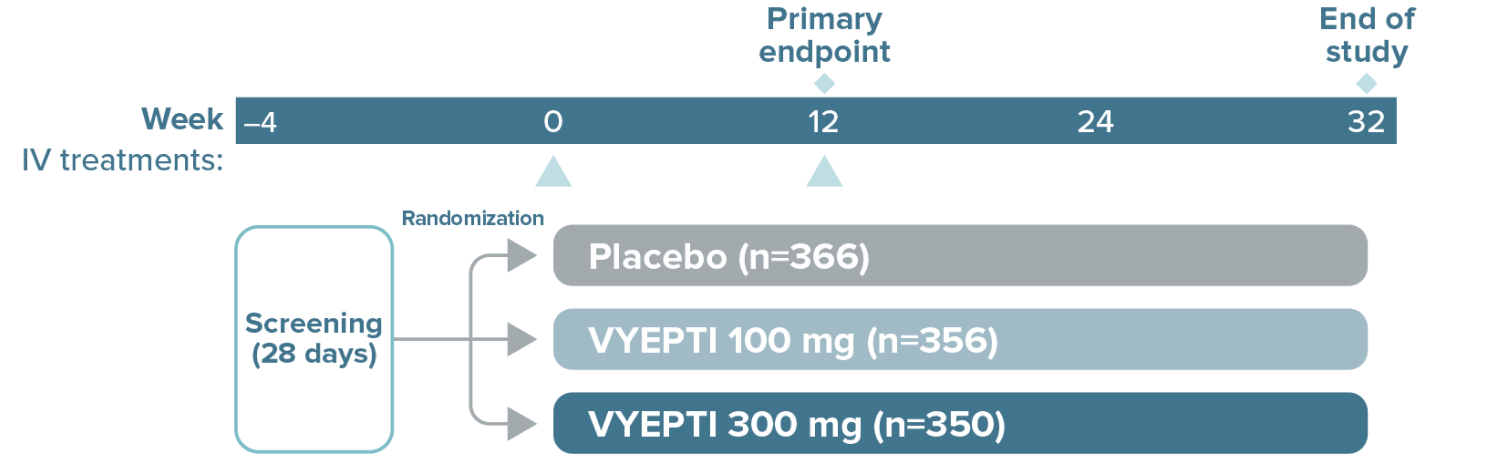
VYEPTI 100 mg (n=356)
Placebo (n=366)
The study included:
- Patients using an established stable regimen of acute migraine or headache prevention medication (except onabotulinumtoxinA)
- Patients with a dual diagnosis of chronic migraine and medication overuse headache attributable to acute medication overuse (triptans, ergotamine, or combination analgesics >10 days per month)
The study excluded:
- Patients using opioids or butalbital-containing products >4 days per month
- Patients with a history of:
- Cardiovascular disease (hypertension, ischemic heart disease)
- Neurological disease
- Cerebrovascular disease
≥75% migraine response rate6*
VYEPTI 100 mg (n=221)
Placebo (n=222)
The study included:
- Patients using concurrent acute migraine or headache medications, including migraine-specific medications (eg, triptans, ergotamine derivatives)
The study excluded patients with a history of:
- Cardiovascular disease (hypertension, ischemic heart disease)
- Neurological disease
- Cerebrovascular disease
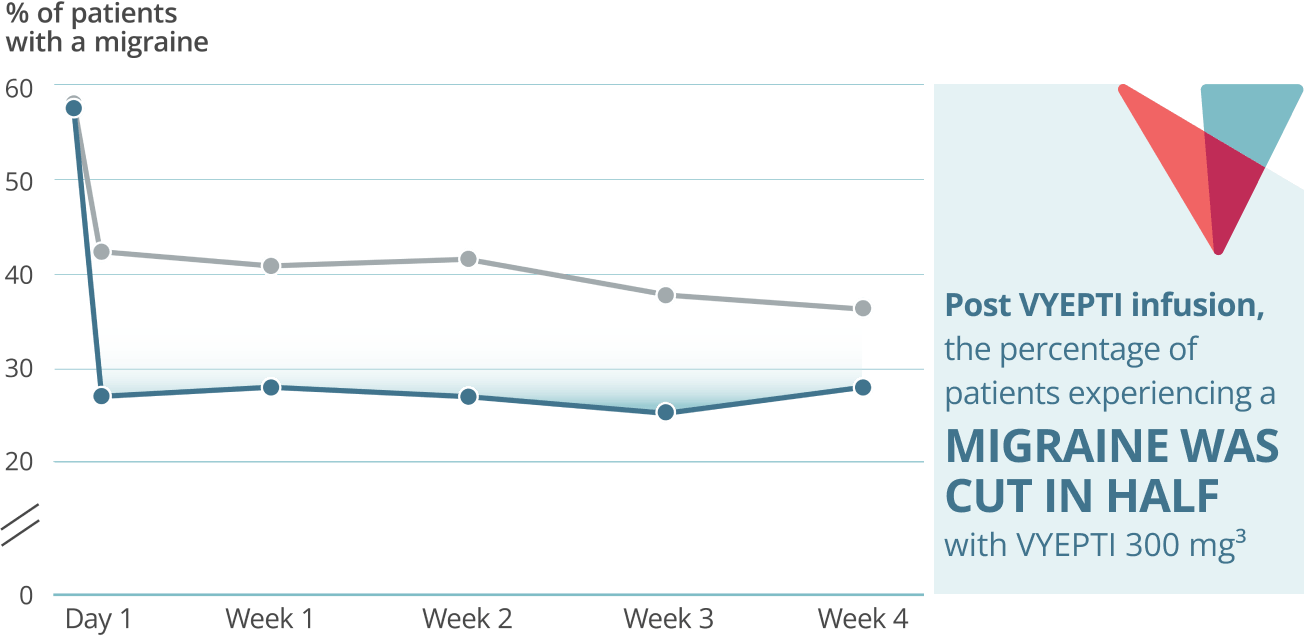
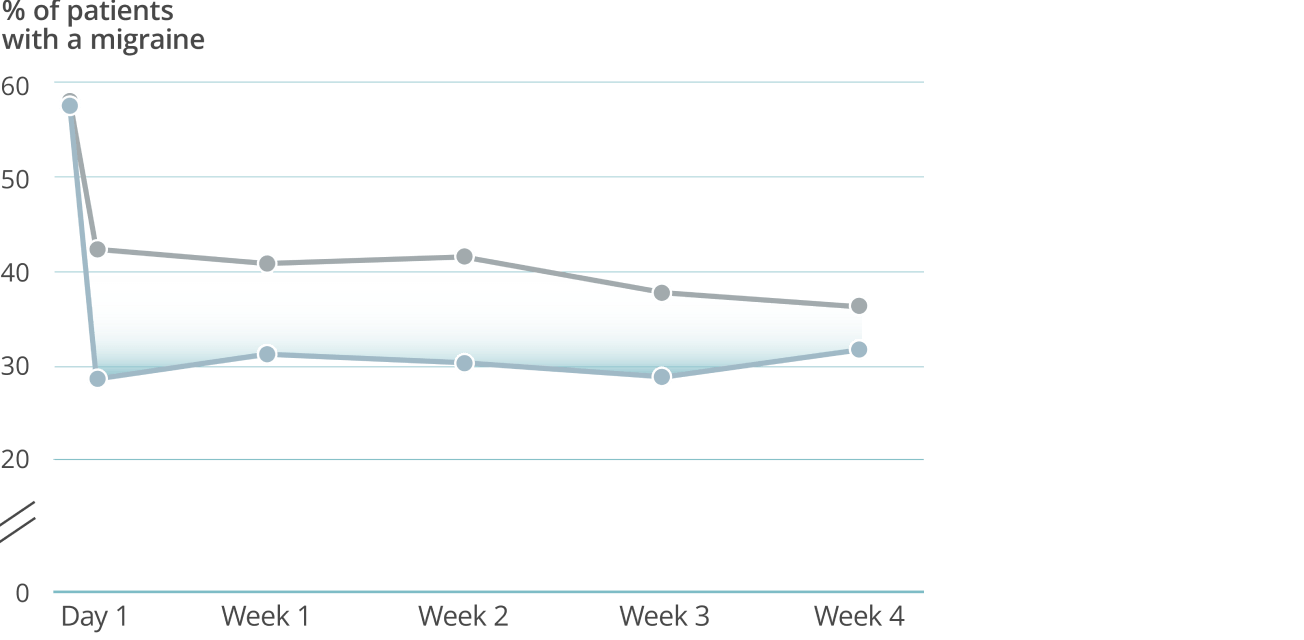
Fast onset: Day 1 post infusion results3†
VYEPTI 100 mg (n=356)
Placebo (n=366)
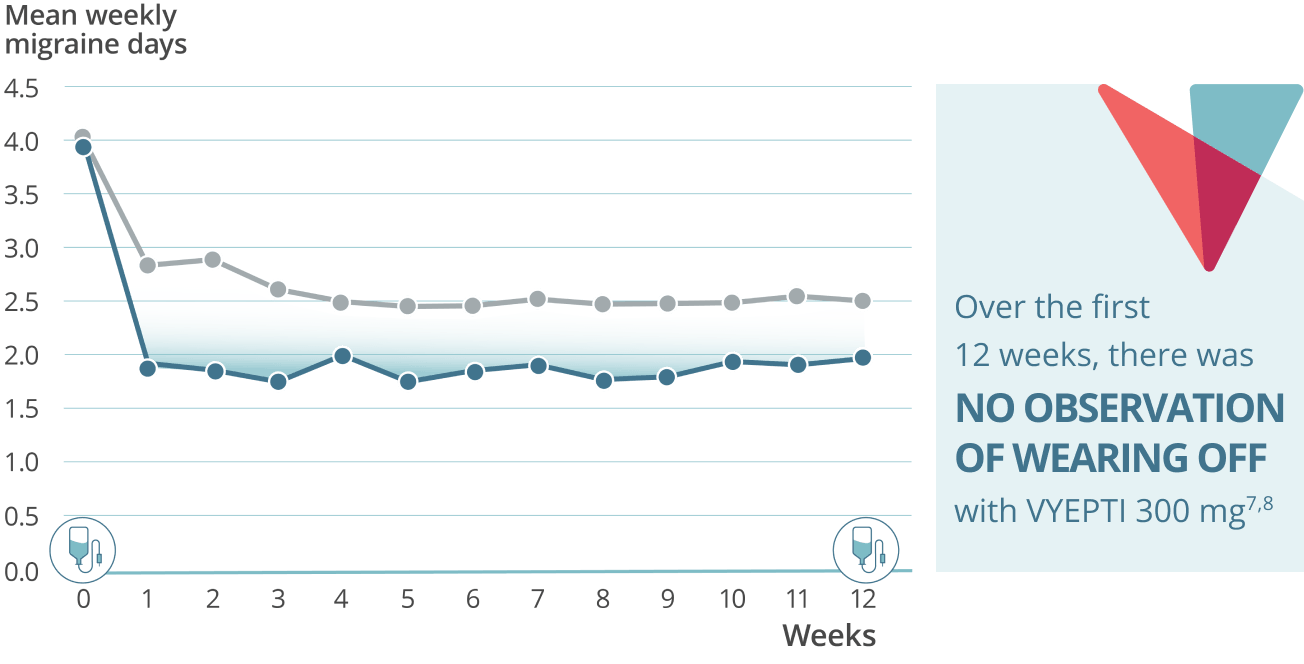
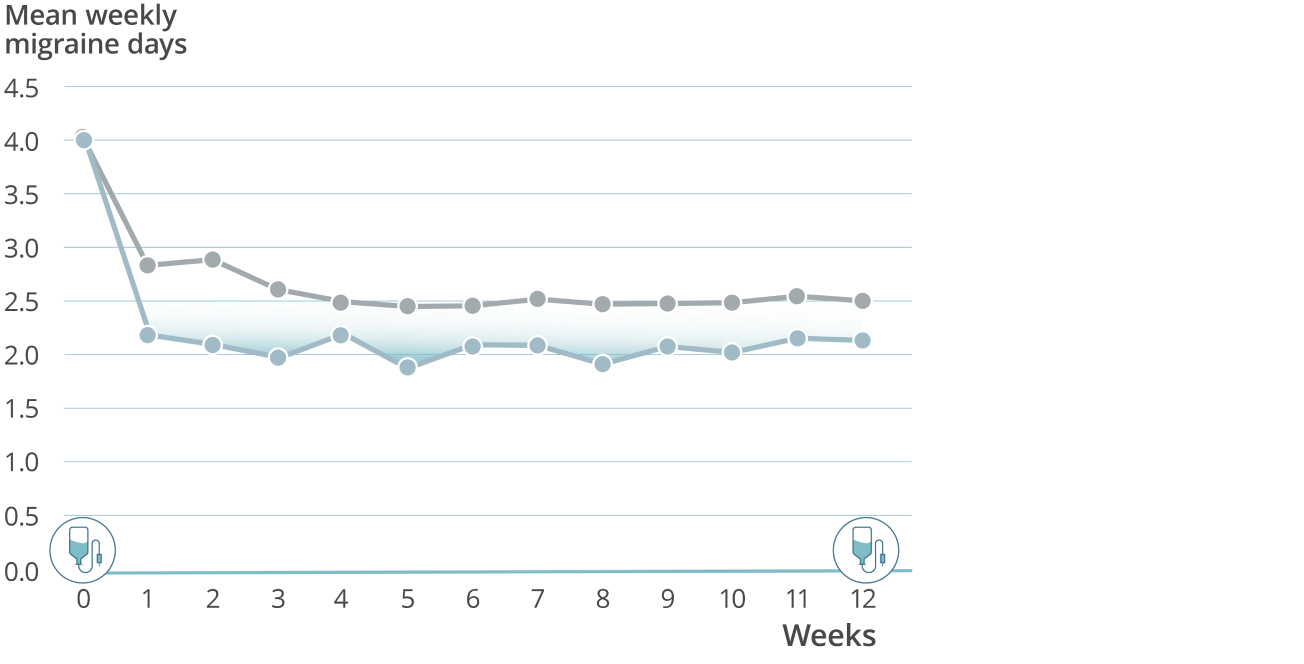
Sustained prevention: Mean weekly migraine days through week 127,8
VYEPTI 100 mg (n=356)
Placebo (n=366)
The study included:
- Patients using an established stable regimen of acute migraine or headache prevention medication (except onabotulinumtoxinA)
- Patients with a dual diagnosis of chronic migraine and medication overuse headache attributable to acute medication overuse (triptans, ergotamine, or combination analgesics >10 days per month)
The study excluded:
- Patients using opioids or butalbital-containing products >4 days per month
- Patients with a history of:
- Cardiovascular disease (hypertension, ischemic heart disease)
- Neurological disease
- Cerebrovascular disease
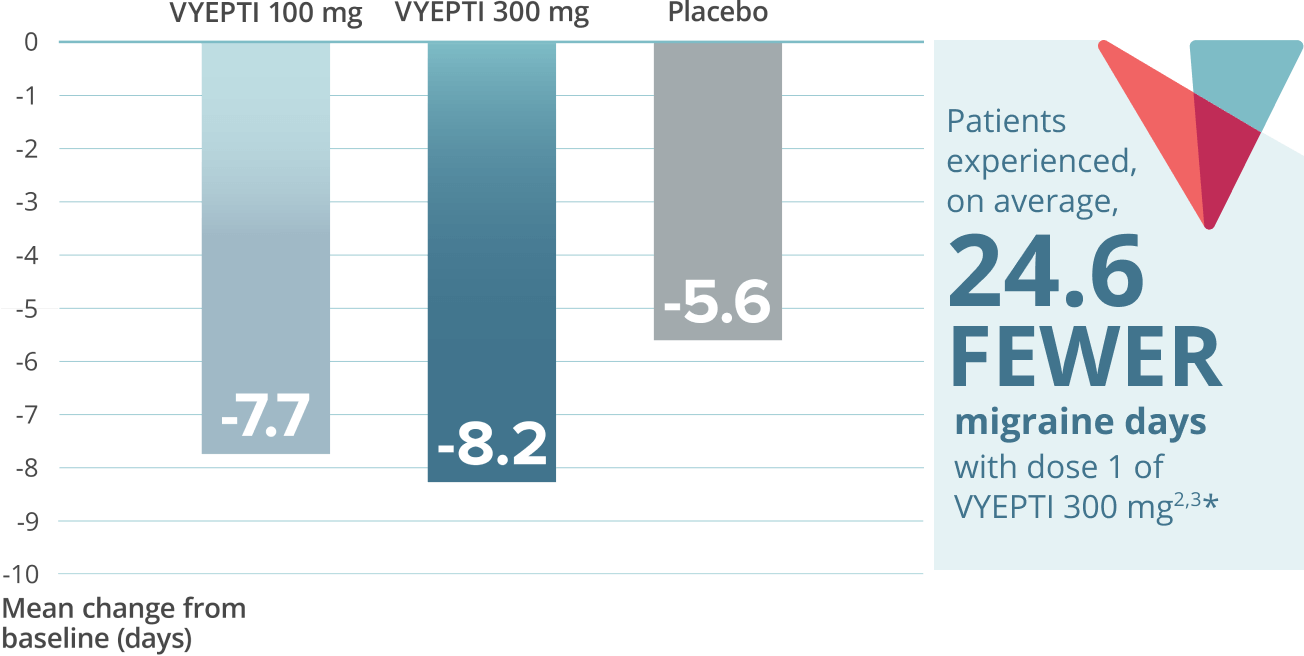
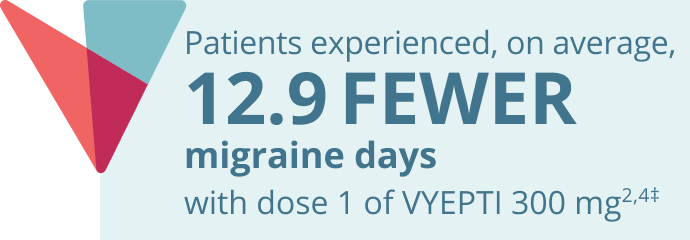
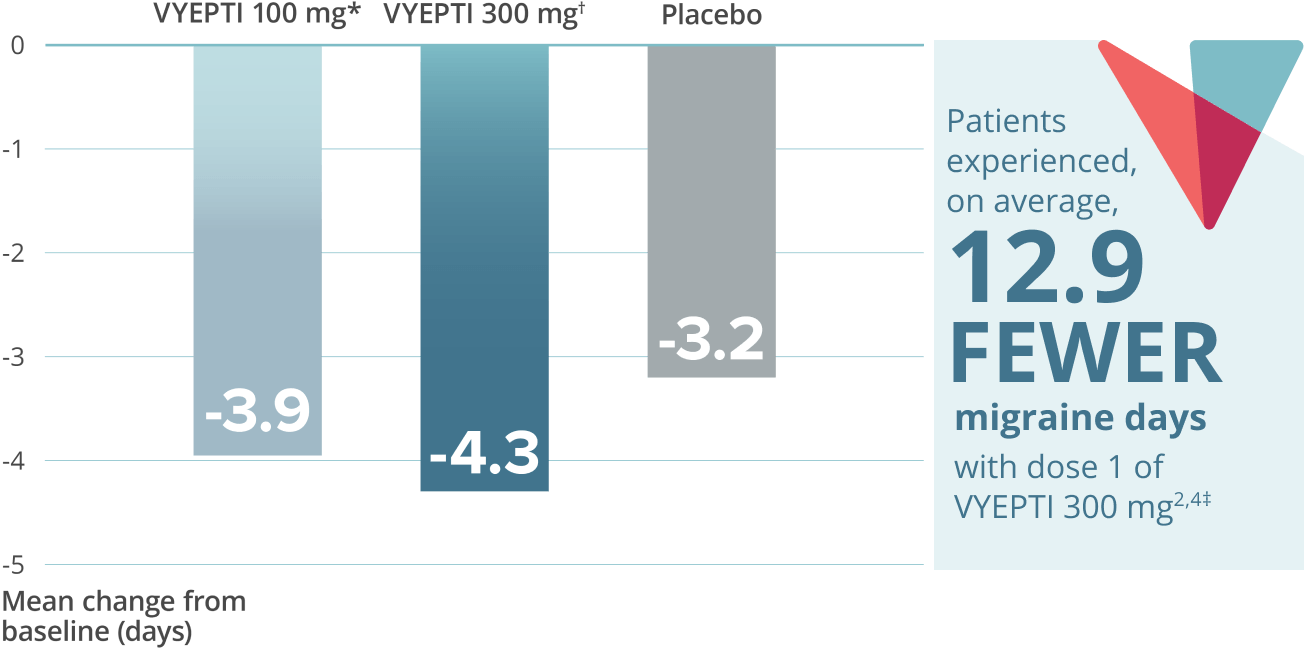
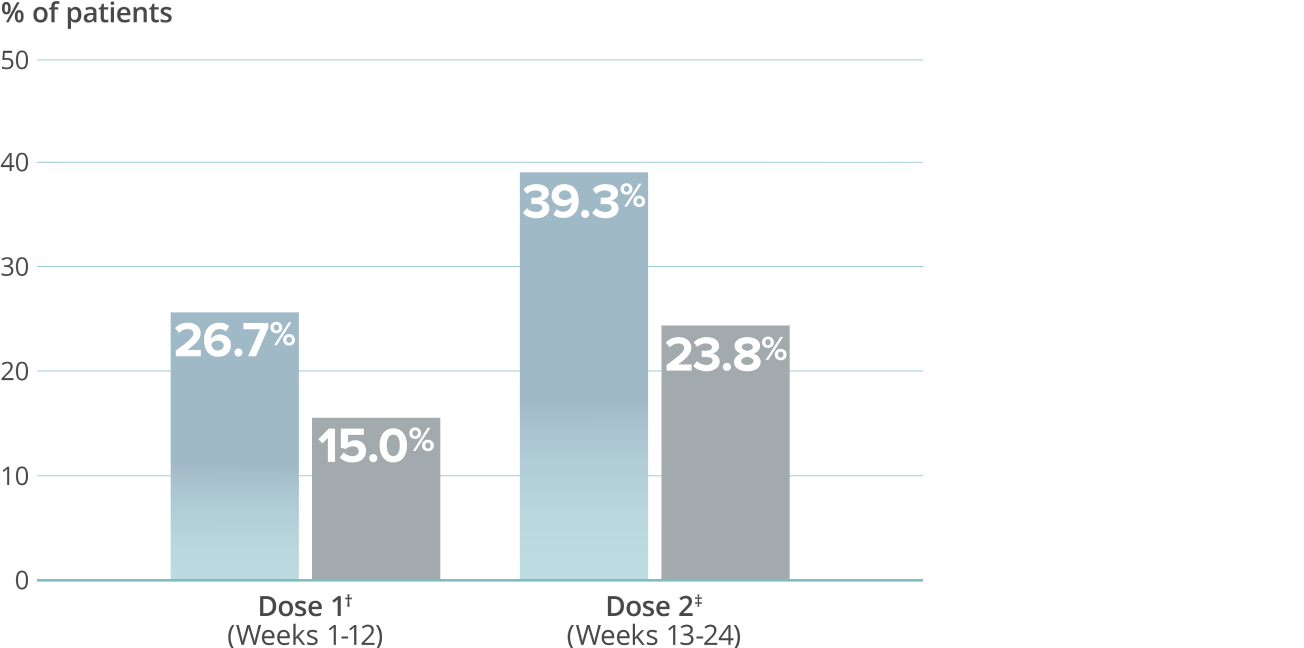
Reduction in mean acute headache medication (AHM) days over two doses5,9*
VYEPTI 100 mg (n=356)
Placebo (n=366)
The study included:
- Patients using an established stable regimen of acute migraine or headache prevention medication (except onabotulinumtoxinA)
- Patients with a dual diagnosis of chronic migraine and medication overuse headache attributable to acute medication overuse (triptans, ergotamine, or combination analgesics >10 days per month)
The study excluded:
- Patients using opioids or butalbital-containing products >4 days per month
- Patients with a history of:
- Cardiovascular disease (hypertension, ischemic heart disease)
- Neurological disease
- Cerebrovascular disease
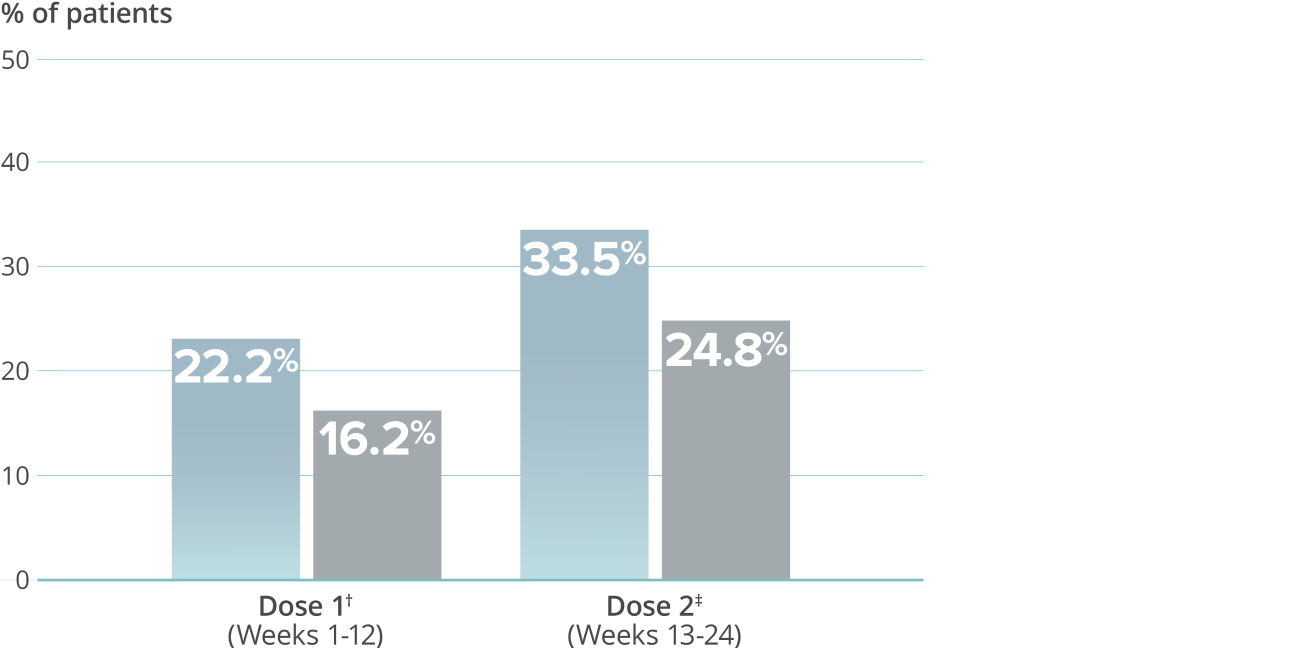
Percentage of patients experiencing severe life impact as measured by HIT-65,9*†
VYEPTI 300 mg (n=356)
Placebo (n=366)
The study included:
- Patients using an established stable regimen of acute migraine or headache prevention medication (except onabotulinumtoxinA)
- Patients with a dual diagnosis of chronic migraine and medication overuse headache attributable to acute medication overuse (triptans, ergotamine, or combination analgesics >10 days per month)
The study excluded:
- Patients using opioids or butalbital-containing products >4 days per month
- Patients with a history of:
- Cardiovascular disease (hypertension, ischemic heart disease)
- Neurological disease
- Cerebrovascular disease
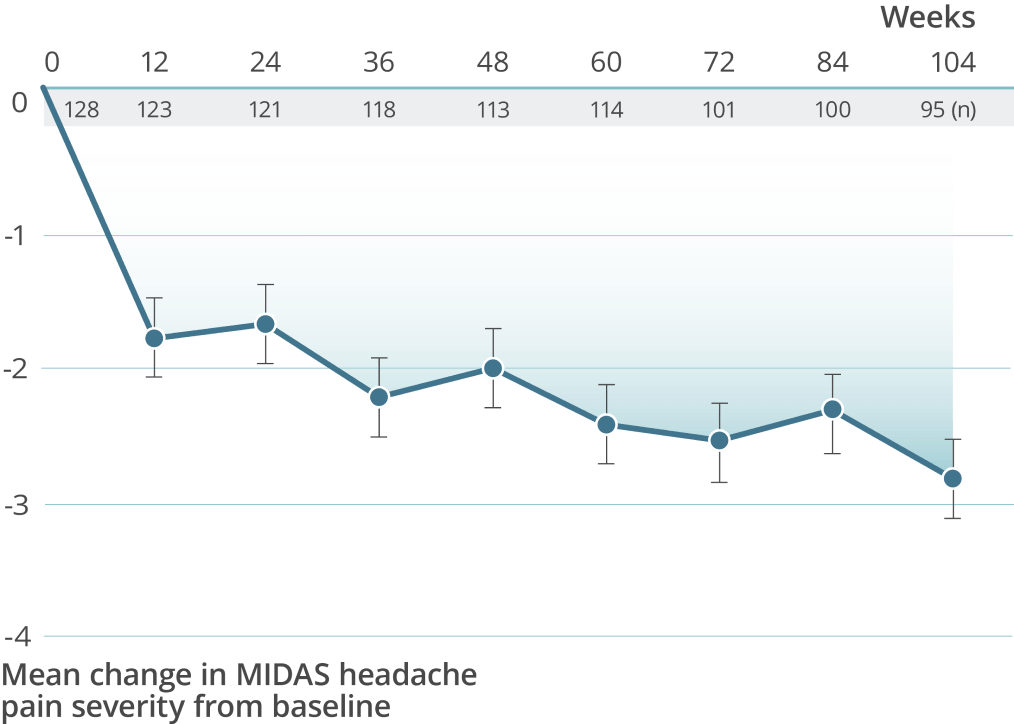
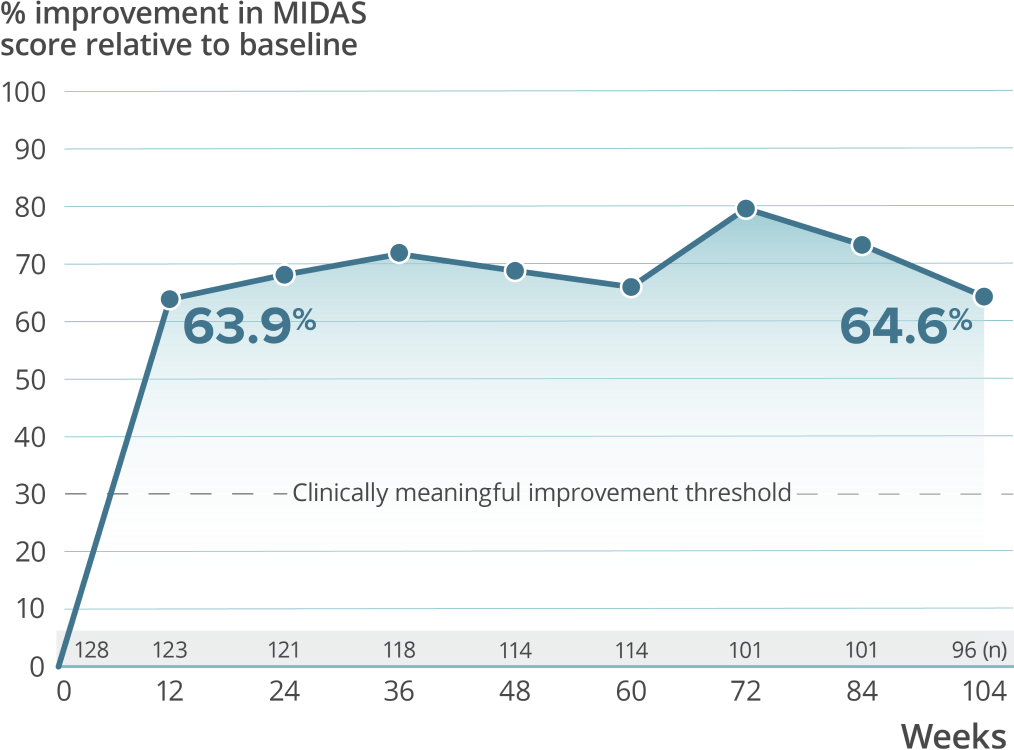
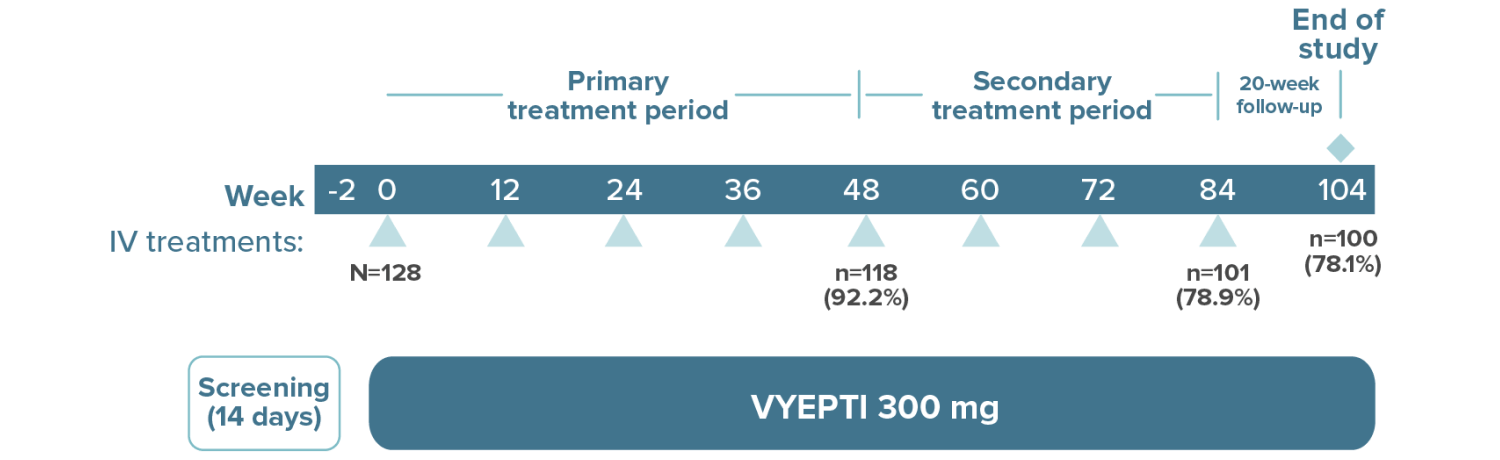
High treatment persistency was demonstrated over 2 years††
††In the 2-year PREVAIL study, a total of 118 patients (92.2%) completed the primary treatment phase (week 48), and 101 (78.9%) completed the secondary (week 84); 100 patients (78.1%) remained on VYEPTI 20 weeks after administration of the final study dose (week 104), and 6.3% discontinued study drug due to adverse events.

of patients treated with VYEPTI 300 mg continued to receive treatment10,11
††In the 2-year PREVAIL study, a total of 118 patients (92.2%) completed the primary treatment phase (week 48), and 101 (78.9%) completed the secondary (week 84); 100 patients (78.1%) remained on VYEPTI 20 weeks after administration of the final study dose (week 104), and 6.3% discontinued study drug due to adverse events.
The study included:
- Adults (18-65 years old) with an ICHD-3β diagnosis of migraine at or before age 50 with ≥12-month history of chronic migraine advised by a healthcare professional to use medication to treat their migraine
- Prior migraine prophylactic medications had to be stable for 3 months prior
- Limited use of barbiturate or prescription opiates was allowed
The study excluded patients who:
- Required botulinum toxin injections for any reason within 4 months prior to screening
- Received any monoclonal antibody targeting the CGRP pathway within 6 months prior to screening
- Had pre-existing significant cardiovascular disease
- Had clinically significant pain syndromes
ICHD, International Classification of Headache Disorders.