From the American Headache Society position statement update, 2024
Real-world studies include both prospective and retrospective studies. Although the evidence provided by these studies is not considered to be of the same quality as randomized control trials (RCTs), they are very useful in that they generally confirm the results of the RCTs regarding efficacy, tolerability, and safety, and they do so within a wide variety of international cohorts, often over longer time periods.1
Explore the VYEPTI pivotal trials and 2-year patient-reported outcomes data to see if VYEPTI could be right for your patients.
Read full clinical publication
For REVIEW, 3 components comprised the study: a structured patient survey intended to measure their satisfaction with VYEPTI, a retrospective chart review, and a semi-structured healthcare provider interview. Study data reflect patients' experiences with migraine treatment and their migraine symptomatology collected across 4 study sites in the US. Data are survey-based and dependent upon patient recall. Descriptive statistics were used; no conclusions can be drawn.2,3
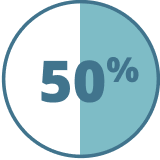
To participate, patients must have completed a minimum of 2 consecutive infusion cycles (≥6-month exposure) with 51% of patients having received 5 or more at the time of the survey. Patients were not excluded based on comorbidities.
The structured patient survey underwent linguistic validation to ensure that patients understood the intent of each question.2
Select baseline characteristics
- Mean age: 49.2 years (median: 49 years)
- Mean time since diagnosis: 15.4 years (median: 12 years)
- Current VYEPTI dose: 57% 300 mg | 23% 100 mg | 19% cannot recall/missing/other
- 100% of respondents had prior preventive treatment: 89% anti-CGRP mAb | 82% OnabotulinumtoxinA | 74% oral preventive | 73% Gepant
Select inclusion criteria
- Over 18 years of age
- Diagnosis of chronic migraine (per the patient’s chart)
- Received ≥2 consecutive VYEPTI infusion cycles
- Ability to complete survey in English
Exclusion criteria
- Treated with VYEPTI in a clinical trial setting
- Enrolled in another clinical trial for migraine
Limitations
- Patient data were survey-based, self-reported, and dependent on patient recall
- Patient population selected based on treatment with VYEPTI regardless of prescribed dosage, suggesting positive responses and tolerability
- No cross-comparisons were conducted among outcome measures for the 100 mg, 200 mg, or 300 mg doses
- Gepants were not excluded, and methodology did not support differentiation between preventive and acute use
- Inherent selection bias in the study could not be mitigated
- Results are descriptive data; no formal statistical analyses were performed
CGRP, calcitonin gene-related peptide; mAb, monoclonal antibody.
VYEPTI from the perspective of your peers
Examining the burden of chronic migraine and a clinical case study
"It’s so important for us to really understand what our patients experience and how we can provide support both emotionally and clinically."
Wade Cooper, DO
Treating chronic migraine, illustrated by a patient case study
"We'll dig deeper into the VYEPTI patient reported outcomes data as reported in the clinical trials."
Peter McAllister, MD FAAN
††Survey consisted of people diagnosed with migraine disease for ≥2 years and who satisfy at least 1 of the following: ≥8 migraine attacks in the prior month, have been prescribed a preventive migraine treatment, or are either currently taking or have previously taken a preventive migraine treatment.
Hear from more patients who were moved to VYEPTI
All real VYEPTI patients on this page were compensated for their time. Patient results may vary